Tracing absorption and emission characteristics of halogen-bonded ion pairs involving halogenated imidazolium species†
Abstract
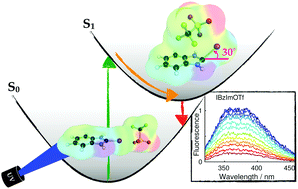
We investigate how the absorption and fluorescence of halogenated imidazolium compounds in acetonitrile solution is influenced by the presence of counterions and the ability to act as halogen-bond donors. Experimental measurements and quantum chemical calculations with correlated wavefunction methods are applied to study three monodentate halogen-bond complexes of iodo-imidazolium, iodo-benzimidazolium and bromo-benzimidazolium cations with triflate counterions, and a bidentate complex of bis(iodo-benzimidazolium) dications with chloride as counterion. The three monodentate complexes with triflate counterions relax after photoexcitation to minima on the S1 potential energy surface where the C–I bond and the I⋯O halogen bond are partially broken. For the bidentate complex with the smaller chloride counterion the halogen-bond interaction stays intact in the S1 minimum that is reached by relaxation from the Franck–Condon point. In a complementing experimental approach, stationary absorption and emission as well as transient fluorescence spectra are recorded for iodo- and bromo-benzimidazolium in acetonitrile. Variation of the counterion, substitution of the iodine by bromine, hydrogen, or methyl, and the comparison to theory allows the identification of spectroscopic signatures and photoinduced dynamics associated with ion-pairing.


 Please wait while we load your content...
Please wait while we load your content...