Formation of water-in-oil microemulsions within a hydrophobic deep eutectic solvent†
Abstract
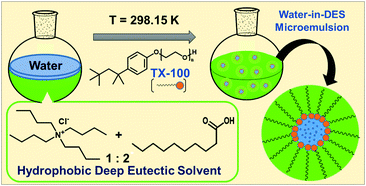
Hydrophobic deep eutectic solvents (DESs) as neoteric, non-toxic, and inexpensive media have the potential to replace organic solvents in various aggregation processes. Conventional water-in-oil microemulsions are formed using mostly environmentally unfavorable toxic organic solvents as the bulk oil phase. Evidence of formation of water-in-DES microemulsions is presented. These novel assemblies are formed using a hydrophobic DES constituted of n-decanoic acid (DA) and tetra-n-butylammonium chloride (TBAC) in 2 : 1 mole ratio, termed TBAC-DA, as the bulk oil phase. It is observed that in the presence of a common and popular non-ionic surfactant Triton X-100 (TX-100), water pools are formed within TBAC-DA under ambient conditions with maximum water loading (w0 = [water]/[TX-100]) of 60 ± 3 for [TX-100] = 300 mM. The formation of the microemulsions is established by using fluorescence probe pyranine, which exhibited the appearance of a band characterizing the un-protonated form of the probe clearly implying onset of water-in-TBAC-DA microemulsion formation. The UV-vis absorbance of CoII further corroborates TX-100-assisted water pool formation within TBAC-DA via the appearance of the band that is assigned to the response of the probe in water. Dynamic light scattering (DLS) measurement suggests average aggregate sizes to be in the range of 72(±4) to 122(±7) nm. These unprecedented water-in-DES microemulsions may have far reaching implications due to their benign nature.
- This article is part of the themed collection: Non-traditional solvent effects in organic reactions


 Please wait while we load your content...
Please wait while we load your content...