Experimental and theoretical assessment of protonated Hoogsteen 9-methylguanine–1-methylcytosine base-pair dissociation: kinetics within a statistical reaction framework†
Abstract
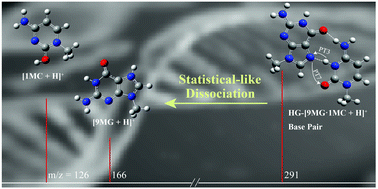
We investigated the collision-induced dissociation (CID) reactions of a protonated Hoogsteen 9-methylguanine–1-methylcytosine base pair (HG-[9MG·1MC + H]+), which aims to address the mystery of the literature reported “anomaly” in product ion distributions and compare the kinetics of a Hoogsteen base pair with its Watson-Crick isomer WC-[9MG·1MC + H]+ (reported recently by Sun et al.; Phys. Chem. Chem. Phys., 2020, 22, 24986). Product ion cross sections and branching ratios were measured as a function of center-of-mass collision energy using guided-ion beam tandem mass spectrometry, from which base-pair dissociation energies were determined. Product structures and energetics were assessed using various theories, of which the composite DLPNO-CCSD(T)/aug-cc-pVTZ//ωB97XD/6-311++G(d,p) was adopted as the best-performing method for constructing a reaction potential energy surface. The statistical Rice–Ramsperger–Kassel–Marcus theory was found to provide a useful framework for rationalizing the dominating abundance of [1MC + H]+ over [9MG + H]+ in the fragment ions of HG-[9MG·1MC + H]+. The kinetics analysis proved the necessity for incorporating into kinetics modeling not only the static properties of reaction minima and transition states but more importantly, the kinetics of individual base-pair conformers that have formed in collisional activation. The analysis also pinpointed the origin of the statistical kinetics of HG-[9MG·1MC + H]+vs. the non-statistical behavior of WC-[9MG·1MC + H]+ in terms of their distinctively different intra-base-pair hydrogen-bonds and consequently the absence of proton transfer between the N1 position of 9MG and the N3′ of 1MC in the Hoogsteen base pair. Finally, the Hoogsteen base pair was examined in the presence of a water ligand, i.e., HG-[9MG·1MC + H]+·H2O. Besides the same type of base-pair dissociation as detected in dry HG-[9MG·1MC + H]+, secondary methanol elimination was observed via the SN2 reaction of water with nucleobase methyl groups.


 Please wait while we load your content...
Please wait while we load your content...
