Probing hyperconjugative aromaticity in 2H-pyrrolium and cyclopentadiene containing group 9 transition metal substituents: bridged carbonyl ligands can enhance aromaticity†
Abstract
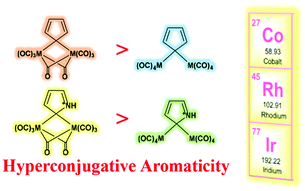
Aromaticity and hyperconjugation are two fundamental concepts in organic chemistry. By combination of the two concepts together, the resulting hyperconjugative aromaticity has attracted considerable attention from both theoretical and computational chemists. However, previous studies are mainly focused on the main group chemistry. For the hyperconjugative aromaticity in the transition metal chemistry, the studies are limited to groups 10 and 11. Here, we demonstrate that hyperconjugative aromaticity can be achieved in 2H-pyrrolium and cyclopentadiene containing group 9 transition metal substituents via density functional theory calculations. More importantly, further studies reveal that the metal–metal bonding interaction between two substituents could reduce hyperconjugative aromaticity, whereas the bridged carbonyl ligands will enhance aromaticity due to the significantly elevated HOMO orbital of the CR2 fragment. All these findings not only extend the scope of the concept of hyperconjugative aromaticity but also are helpful to develop the chemistry of metalla-aromatics.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...