Transition anionic complex in trihexyl(tetradecyl)phosphonium-bis(oxalato)borate ionic liquid – revisited†
Abstract
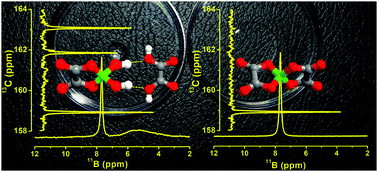
It was found that Li[BOB]·nH2O salts were not readily suitable for the synthesis of high-purity orthoborate-based tetraalkylphosphonium ionic liquids, as exemplified here for trihexyl(tetradecyl)phosphonium bis(oxalato)borate, [P6,6,6,14][BOB]; along with [BOB]−, a metastable transition anionic complex (TAC) of dihydroxy(oxalato)borate with oxalic acid, [B(C2O4)(OH)2·(HOOC–COOH)]−, was also formed and passed into the ionic liquid in the course of the metathesis reaction with trihexyl(tetradecyl)phosphonium chloride. On the contrary, Na[BOB] was found to be a more suitable reagent for the synthesis of this IL, because [BOB]− anions safely passed into the final IL without hydrolysis, when metathesis reactions were performed using aqueous-free media. Since ultra-pure Na[BOB] is not commercially available, in this work, a preparation protocol for ultra-pure (>99%) Na[BOB] was developed: (i) molar ratios of boric and oxalic acids were optimised to minimise boron-containing impurities, (ii) the Na[BOB] product was thoroughly purified by sequential washing of a fine powder product in hot acetonitrile and ethanol and (iii) characterised using powder X-ray diffraction and solid-state 11B MAS NMR spectroscopy. The physico-chemical properties of the prepared boron-impurity-free IL, i.e., its density, viscosity, electric conductivity, glass-transition temperature and thermal stability, were found to be significantly different from those of the previously reported [P6,6,6,14][BOB], containing ca. 45 mol% of TAC, [B(C2O4)(OH)2·(HOOC–COOH)]−. It was found that a high-purity [P6,6,6,14][BOB] prepared in this work has a considerably lower viscosity, a higher viscosity index and a wider electro-chemical window (ECW) compared to those of the sample of [P6,6,6,14][BOB] with ca. 45 mol% of TAC. Interestingly, [B(C2O4)(OH)2·(HOOC–COOH)]− in the latter sample almost completely transformed into [BOB]− anions upon heating of the IL sample at 413 K for 1 hour, as confirmed using both 11B and 13C NMR. Therefore, in this work, apart from a well-optimised synthetic protocol for boron-impurity-free [P6,6,6,14][BOB], implications of boron-containing transition anionic complexes in tetraalkylphosphonium-orthoborate ILs used in different applications were highlighted.


 Please wait while we load your content...
Please wait while we load your content...