Proton conduction in hydronium solvate ionic liquids affected by ligand shape†
Abstract
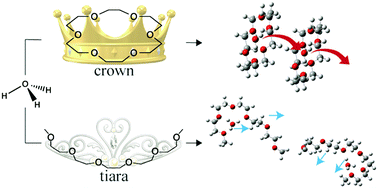
We investigated the ligand dependence of the proton conduction of hydronium solvate ionic liquids (ILs), consisting of a hydronium ion (H3O+), polyether ligands, and a bis[(trifluoromethyl)sulfonyl]amide anion (Tf2N−; Tf = CF3SO2). The ligands were changed from previously reported 18-crown-6 (18C6) to other cyclic or acyclic polyethers, namely, dicyclohexano-18-crown-6 (Dh18C6), benzo-18-crown-6 (B18C6) and pentaethylene glycol dimethyl ether (G5). Pulsed-field gradient spin echo nuclear magnetic resonance results revealed that the protons of H3O+ move faster than those of cyclic 18C6-based ligands but as fast as those of acyclic G5 ligands. Based on these results and density functional theory calculations, we propose that the coordination of a cyclic ether ligand to the H3O+ ion is essential for fast proton conduction in hydronium solvate ILs. Our results attract special interest for many electro- and bio-chemical applications such as electrolyte systems for fuel cells and artificial ion channels for biological cells.


 Please wait while we load your content...
Please wait while we load your content...