Thermodynamic and structural study of DMPC–alkanol systems†
Abstract
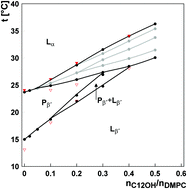
The thermodynamic and structural behaviors of lamellar dimyristoylphosphatidylcholine-alkanol (abbreviation DMPC–CnOH, n = 8–18 is the even number of carbons in the alkyl chain) systems were studied by using DSC and SAXD/WAXD methods at a 0–0.8 CnOH : DMPC molar ratio range. Up to n ≤ 10 a significant biphasic effect depending on the main transition temperature tm on the CnOH concentration was observed. Two breakpoints were revealed: turning point (TP), corresponding to the minimum, and threshold concentration (cT), corresponding to the end of the biphasic tendency. These breakpoints were also observed in the alkanol concentration dependent change in the enthalpy of the main transition ΔHm. In the case of CnOHs with n > 10 we propose a marked shift of TP and cT to very low concentrations; consequently, only increase of tm is observed. A partial phase diagram was constructed for a pseudo-binary DMPC–C12OH system. We suggest a fluid–fluid immiscibility of the DMPC–C12OH system above cT with a consequent formation of domains with different C12OH contents. At a constant CnOH concentration, the effects of CnOHs on ΔHm and bilayer repeat distance were found to depend predominantly on the mismatch between CnOH and lipid chain lengths. Observed effects are suggested to be underlined by a counterbalancing effect of interchain van der Waals interactions and headgroup repulsion.


 Please wait while we load your content...
Please wait while we load your content...