Chaotic oscillations, dissipation and mirror symmetry breaking in a chiral catalytic network
Abstract
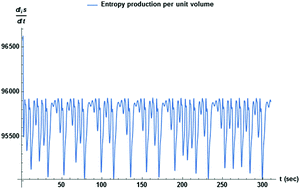
Catalytic reaction networks consist of molecular arrays interconnected by autocatalysis and cross-catalytic pathways among the reactants, and serve as bottom-up models for the design and understanding of molecular evolution and emergent phenomena. An important example of the latter is the emergence of homochirality in biomolecules during chemical evolution. This chiral symmetry breaking is triggered by bistability and bifurcation in networks of chiral replicators. Spontaneous mirror symmetry breaking (SMSB) results from hypercyclic connectivity when the chirality and enantioselectivity of the replicators are taken into account. Heretofore, SMSB has been generally understood as involving chemical transformations yielding scalemic outcomes as non-equilibrium steady states (NESS). Here, in marked contrast, we consider the chaotic regime, in which steady states do not exist. The dissipation, or entropy production, is chaotic as is the exchange entropy. The rate of change of the total system entropy, governed by the entropy balance equation, is also chaotic. Subsequent to the mirror symmetry breaking transition, the time averaged entropy production is minimized in the final chaotic chiral state with respect to the former chaotic racemic state. The chemical forces (i.e., the affinities) evolve in time so as to lower the sum of the entropy production and the exchange entropy, in compliance with the general evolution criterion extended to reaction networks subject to volumetric open flow.


 Please wait while we load your content...
Please wait while we load your content...