Predissociation spectra of the 35Cl−(H2) complex and its isotopologue 35Cl−(D2)†
Abstract
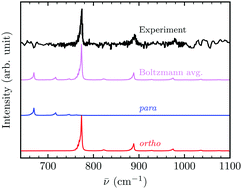
The predissociation spectra of the 35Cl−(H2) and 35Cl−(D2) complexes are determined within an accurate quantum approach and compared to those recently measured in an ionic trap at 8 K and 22 K. The calculations are performed using an existing three-dimensional potential energy surface. A variational approach is used for the accurate quantum calculations of the rovibrational bound states. Several methods are compared for the search and the characterization of the resonant states. A good agreement between the calculated and measured spectra is obtained, despite a slight shift to the red of the calculated spectra. The comparison shows that only the ortho or para contribution is observed in the measured 35Cl−(H2) or 35Cl−(D2) spectrum, respectively. Quantum numbers are assigned to the rovibrational resonant states. It demonstrates that the main features observed in the measured predissociation spectra correspond to a progression in the intermonomer vibrational stretching mode.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...