Intriguing structural, bonding and reactivity features in some beryllium containing complexes†
Abstract
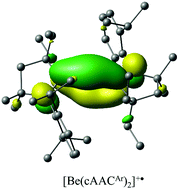
Although the toxicity of beryllium compounds causes impediments in experiments involving them, beryllium chemistry has seen a recent upsurge of interest and considerable progress. Computations play a very important complementary role in analyzing the structure, stability and bonding of these compounds. In this perspective article, we highlighted our contribution to beryllium chemistry which is either completely through theoretical results or sometimes supported by experimental findings. It starts with the smallest 2π aromatic system, Be32−, which also exhibits rare bond-stretch isomerism. Furthermore, its reactivity towards different transformations is mentioned. Because of the ability of beryllium to attain a high ionic potential, the beryllium center in an appropriate situation can act as an excellent Lewis acid which is utilized to bind noble gas (Ng) atoms, carbon monoxide and dinitrogen through donor–acceptor types of interactions. We made several efforts to have strong Ng–Be bonds which led us to NgBeNCN that is recorded to have the strongest Ng–Be bond among the neutral Ng–Be complexes reported so far. Significant dinitrogen activation was also achieved in (NN)2Be(η2-N2) and OCBeNN complexes. In the latter case, a complete cleavage of the N–N bond producing the most stable NBeNCO molecule has occurred. We also found viable M2(NHBMe)2 (M = Be, Mg) complexes having unusual bonding where the interacting fragments are best described as the neutral M2 and (NHBMe)2 but M2 still possesses a single bond. We finally discussed the complex comprising an unusual Be(I) oxidation state, [BeI(cAACAr)2]+˙ and di-ortho-beryllated carbodiphosphorane exhibiting Be⇇C double dative bonds.
- This article is part of the themed collection: PCCP Perspectives


 Please wait while we load your content...
Please wait while we load your content...