TD-DFT and CC2 insights into the dual-emissive behaviour of 2-(2′-hydroxyphenyl)oxazoles core and their derivatives†
Abstract
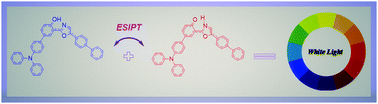
Two efficient excited state intramolecular proton transfer (ESIPT) dyes based on the hydroxyphenyl-oxazole core and containing one or two triphenylamine donor groups are explored with theoretical tools. These compounds are known to show clear experimental dual emission behaviour, leading to nearly pure white-light emission for one derivative. To probe the excited state properties, we use both Time Dependent Density Functional Theory (TD-DFT) and post Hartree–Fock methods [ADC(2) and CC2] coupled to different solvent models to describe polarisation effects. After validating our theoretical protocol on the two known systems, we design 14 new derivatives with different substitution patterns to quantify the impact of electron accepting and donating groups on the fluorescence spectrum and the ESIPT mechanism. We show that the selected protocol delivers accurate spectroscopic values for the two experimentally-characterised structures, and more importantly, that the relative stabilisation of the keto tautomer depends on the substitution side. Adding donor or acceptor groups to the ESIPT donor moiety favours the formation of the keto form, whereas when placed on the ESIPT accepting side, they tend to preclude ESIPT. Moreover, combining two donor or acceptor substituents generally results in similar ESIPT behaviour as single substitution on one of the two sides: simple additive rules do not apply.


 Please wait while we load your content...
Please wait while we load your content...