Are all planar and quasi-planar boron clusters aromatic? Counter examples of island or global π antiaromaticity from chemical bonding analysis
Abstract
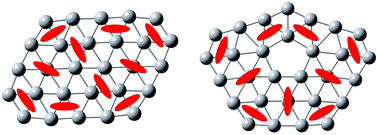
Boron is an electron-deficient element. The flatland of planar or quasi-planar (2D) boron clusters is believed to possess aromaticity for all members, which remains a fundamental issue in debate in boron chemistry. Using a selected set of D2h B62−, C2h B282−, and C2v B29− clusters as counter examples, we shall present computational evidence for global or island π antiaromaticity in 2D boron clusters. The latter two are flattened for the purpose of clarity, which model their quasi-planar C2 or Cs monoanion clusters observed in prior gas-phase experiments. Chemical bonding in the clusters is elucidated collectively on the basis of canonical molecular orbital (CMO) analysis, adaptive natural density partitioning (AdNDP), electron localization functions (ELFs), and localized molecular orbital (LMO) analysis. These results are complementary to each other and yet highly coherent. As a quantitative indicator, nucleus-independent chemical shifts (NICSs) are calculated at selected specific points in the clusters, which help differentiate between π aromaticity and antiaromaticity. Intriguingly, triangular sites in the same boron cluster can be aromatic, antiaromatic, or nonaromatic, despite the fact that they are physically indistinguishable. The phenomenon is understood in analogy to hydrocarbons and polycyclic aromatic hydrocarbons (PAHs). Even perfect sheet-like boron clusters are convertible to the PAH analogous systems. This work provides compelling examples for global and island π antiaromaticity in the 2D boron clusters.


 Please wait while we load your content...
Please wait while we load your content...