New force field for GCMC simulations of D2/H2 quantum sieving in pure silica zeolites
Abstract
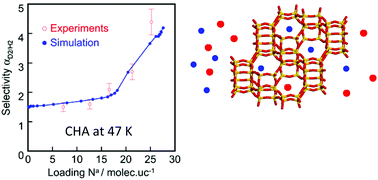
We report a study on adsorption and coadsorption of H2 and D2 in FAU, MFI and CHA pure silica zeolites having different pore sizes and shapes. Adsorption capacities, selectivities, enthalpies and entropies are determined by combining experiments and GCMC simulations. We show that the force fields available in the literature cannot predict the adsorption equilibria below 77 K with sufficient accuracy. Here we propose a new force field adjusted by using our experimental data obtained for the pure silica MFI zeolite at 65 K and 77 K. With this new force field, it is possible to predict the adsorption and coadsorption equilibria on the three zeolite structures in a temperature range between 47 and 77 K with satisfactory precision. We corroborate that the step appearing on the single adsorption isotherms in CHA is the result of a molecular rearrangement of the adsorbed phase due to the apparition of a new adsorption site characterized by weaker interactions of H2 with the adsorbent. We conclude that the quantum sieving of H2 and D2 not only depends on the pore size but also on the pore shape, in particular, at high loading when the confinement effects become important.


 Please wait while we load your content...
Please wait while we load your content...