Hydrophobic solvation increases thermal conductivity of water†
Abstract
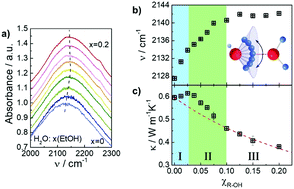
The interaction of water with small alcohols can be used as a model for understanding hydrophobic solvation of larger and more complex amphiphilic molecules. Despite its apparent simplicity, water/ethanol mixtures show important anomalies in several of their properties, like specific heat or partial molar volume, whose precise origin are still a matter of debate. Here we report high-resolution thermal conductivity, compressibility, and IR-spectroscopy data for water/ethanol solutions showing three distinct regions of solvation, related to changes in the H-bond network. Notably, the thermal conductivity shows a surprising increase of ≈3.1% with respect to pure water at dilute concentrations of ethanol (x = 0.025), which suggests a strengthening of H-bond network of water. Our results prove that the rate of energy transfer in water can be increased by hydrophobic solvation, due to the cooperative nature of the H-bond network.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...