The reaction of O(3P) with alkynes: a dynamic and computational study focusing on formyl radical production†
Abstract
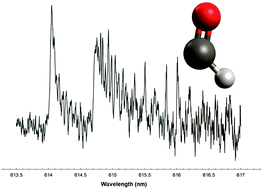
Production of formyl radical, HCO, from reactions of O(3P) with alkynes (acetylene, propyne, 1-butyne, and 1-pentyne) has been investigated using cavity ringdown laser absorption spectroscopy (CRDLAS) and computational methods. No HCO was detected from reaction with acetylene, while the amount of HCO increased for propyne and 1-butyne, dropping off somewhat for 1-pentyne. These results differ from trends previously observed for reactions of O(3P) with alkenes, which exhibit the largest HCO production for the smallest alkene and drop off as the alkene size increases. Computational studies employing density functional and coupled cluster methods have been employed to investigate the triplet and singlet state pathways for HCO production. Because intersystem crossing (ISC) has been shown to be important in these processes, the minimum energy crossing point (MECP) between the triplet and singlet surfaces has been studied. We find the MECP for propyne to possess C1 symmetry and to lie lower in energy than previous studies have found. Natural Bond Orbital and Natural Resonance Theory analyses have been performed to investigate the changes in spin density and bond order along the reaction pathways for formation of HCO. Explanations are suggested for the trend in HCO formation observed for the alkynes. The trend in alkyne HCO yield also is compared and contrasted with the trend previously observed for the alkenes.


 Please wait while we load your content...
Please wait while we load your content...