Theoretical study of metal/silica interfaces: Ti, Fe, Cr and Ni on β-cristobalite†
Abstract
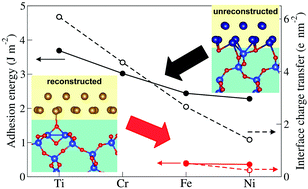
The understanding of interfacial effects and adhesion at oxide-metal contacts is of key importance in modern technology. Metal-silica interfaces specifically are relevant in electronics, catalysis and nanotechnology. However, adhesion at these interfaces is hindered by a formation of siloxane rings on the silica surface which saturate the dangling bonds at stoichiometric terminations. In this context, we report a thorough density functional theory study of the interaction between β-cristobalite and selected 3d transition metals under different oxygen conditions. For any given interface stoichiometry, we find a progressive decrease of the metal/silica interaction along the series, following the increase of metal electronegativity. Crucially, in presence of early transition metals (Ti or Cr) the surface siloxane rings are spontaneously broken, allowing for strong adhesion. Late transition metals interact only weakly with reconstructed surfaces, similarly to what was found for zinc. In absence of reconstruction, stoichiometric silica/metal contacts behave similarly to alumina/metal contacts, but display larger interactions across the interface. Based on these results, we show that early transition metal or stainless steel buffers can significantly improve the weak adhesion between silica and zinc, responsible for a poor performance of anti-corrosive galvanic zinc coatings on modern advanced high strength steels.


 Please wait while we load your content...
Please wait while we load your content...