Transient species of esculetin produced in pulse radiolysis: experimental and quantum chemical investigations†
Abstract
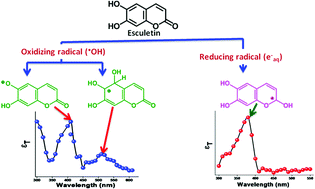
Radiation chemical studies of esculetin (E), a dihydroxycoumarin derivative, were performed using a pulse radiolysis technique employing kinetic spectrometer and quantum chemical calculations. Both the oxidizing radicals, hydroxyl (˙OH) and azide (N3˙) radicals, and the reducing radical hydrated electron (eaq−) and hydrogen atom (H˙) reactions of E were used for the present study. The reaction of ˙OH and N3˙ radicals with E produced transients that absorbed at 410 nm; additionally, another broad band at 510 nm was observed for the ˙OH radical reaction. The reaction of ˙OH radicals with E formed the phenoxyl radical and ˙OH-adducts. It was revealed that 32% of the ˙OH radical reaction products of E were oxidizing in nature and 47% were reducing in nature. The carbonyl group of E was reduced by eaq− and subsequently converted to a neutral radical adduct upon protonation. Similarly, the H˙ atom reaction with E yielded a neutral adduct along with H˙ atom addition products. The transient product absorbed at 380 nm when E was reduced by eaq− and the H˙ atom; additionally, the H˙ atom addition product absorbed at 500 nm. In the case of E, the oxidizing radicals were reactive towards the aromatic ring and the phenolic OH group, whereas the reducing radicals were reactive towards the carbonyl group of E. Quantum chemical calculations using DFT and TD-DFT methods have supported the experimental observation. There was good agreement between the experimental and theoretical data on a number of occasions. Based on the energetics of the transients, it was suggested that the addition products were exothermic in nature. In the addition reaction with the ˙OH radical, there was a slight increase in the C–C bond length adjacent to the addition site compared to the remaining bonds. During the reduction process through the carbonyl group, the ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond length was increased from 1.221 Å to 1.358 Å. There was an excellent correlation between the calculated and experimentally observed absorption maximum for the oxidized product of E. Overall, these redox studies may find application in developing hydroxycoumarin derivatives as an antioxidant or as an electron transporting agent in biochemical processes. In addition, this information will be helpful for understanding the mechanism of removing pollutant dyes by advanced oxidation processes.
O bond length was increased from 1.221 Å to 1.358 Å. There was an excellent correlation between the calculated and experimentally observed absorption maximum for the oxidized product of E. Overall, these redox studies may find application in developing hydroxycoumarin derivatives as an antioxidant or as an electron transporting agent in biochemical processes. In addition, this information will be helpful for understanding the mechanism of removing pollutant dyes by advanced oxidation processes.


 Please wait while we load your content...
Please wait while we load your content...