Microsolvation of ethyl carbamate conformers: effect of carrier gas on the formation of complexes†
Abstract
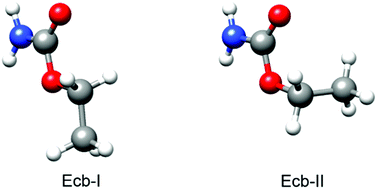
Microsolvated complexes of ethyl carbamate (urethane) with up to three water molecules formed in a supersonic expansion have been characterized by high-resolution microwave spectroscopy. Both chirped-pulse and cavity Fourier transform microwave spectrometers covering the 2–13 GHz frequency range have been used. The structures of the complexes have been characterized and show water molecules closing sequential cycles through hydrogen bonding with the amide group. As is the case in the monomer, the ethyl carbamate–water complexes exhibit a conformational equilibrium between two conformers close in energy. The interconversion barrier between both forms has been studied by analyzing the spectra obtained using different carrier gas in the expansion. Complexation of ethyl carbamate with water molecules does not appear to significantly alter the potential energy function for the interconversion between the two conformations of ethyl carbamate.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...