The multifaceted effects of DMSO and high hydrostatic pressure on the kinetic constants of hydrolysis reactions catalyzed by α-chymotrypsin†
Abstract
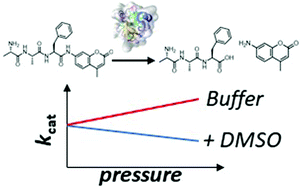
The use of cosolvents and high hydrostatic pressure (HHP) has been described as an efficient means to modulate the stability of enzymes and their catalytic activity. Cosolvents and pressure can lead to increased reaction rates without affecting the stability of the enzyme. Here, we studied the combined effects of one of the most used organic cosolvents, dimethyl sulfoxide (DMSO), and HHP to reveal their combined effect on the kinetic constants of an α-chymotrypsin-catalyzed peptide hydrolysis reaction. The Michaelis constant and the turnover number of the reaction respond differently to the two variables, and we observed an opposite effect of hydrostatic pressure and the dipolar cosolvent DMSO on the kinetic parameters. The results could be rationalized by determining the volume diagram of the reaction at the different solution conditions. In our case, the use of high hydrostatic pressure in concert with DMSO does not lead to an improvement of the enzymatic activity. However, the advantages of DMSO and HHP to increase the temperature stability of the enzyme and to increase the solubility of more hydrophobic substrates could still be useful.
- This article is part of the themed collection: Non-traditional solvent effects in organic reactions


 Please wait while we load your content...
Please wait while we load your content...