Free electron laser infrared action spectroscopy of nitrous oxide binding to platinum clusters, Ptn(N2O)+†
Abstract
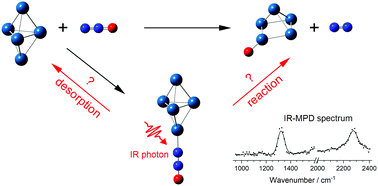
Infrared multiple-photon dissociation spectroscopy has been applied to study Ptn(N2O)+ (n = 1–8) clusters which represent entrance-channel complexes on the reactive potential energy surface for nitrous oxide decomposition on platinum. Comparison of spectra recorded in the spectral region 950 cm−1 to 2400 cm−1 with those simulated for energetically low-lying structures from density functional theory shows a clear preference for molecular binding via the terminal N atom, though evidence of O-binding is observed for some cluster sizes. Enhanced reactivity of Ptn+n ≥ 6 clusters towards N2O is reflected in the calculated reactive potential energy surfaces and, uniquely in the size range studied, Pt6(N2O)+ proved impossible to form in significant number density even with cryogenic cooling of the cluster source. Infrared-driven N2O decomposition, resulting in the formation of cluster oxides, PtnO+, is observed following vibrational excitation of several Ptn(N2O)+ complexes.


 Please wait while we load your content...
Please wait while we load your content...