Decomposition of the simplest ketohydroperoxide in the ozonolysis of ethylene†
Abstract
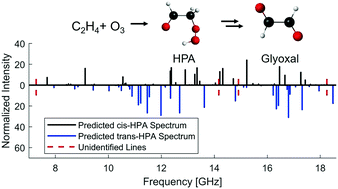
Hydroperoxides from the ozonolysis of alkenes, in addition to Criegee intermediates, have been proposed as an atmospheric source of OH radicals in the absence of sunlight, but have remained largely elusive due to their reactivity. A weak peroxide bond enables facile OH elimination, and subsequent β-scission can lead to a variety of decomposition products depending on the nature of the peroxide. In this paper we explore this process theoretically for the simplest ketohydroperoxide, hydroperoxyacetaldehyde (HPA), which is believed to be formed in the ozonolysis of ethylene. Despite it being the most stable C2H4O3 species in this reaction scheme, lower in energy than the starting materials by around 100 kcal mol−1, HPA has only been directly observed once in the ozonolysis of ethylene by photoionization mass spectrometry appearance energy. Here we report predictions of the rotational spectrum of HPA conducted in support of microwave spectroscopy experiments. We suggest a new dissociation path from HPA to glyoxal [HOOCH2CHO → HCOCH2O + OH → CHOCHO + H], supported by thermochemical calculations. We encourage the search for glyoxal using complementary experimental methods, and suggest possible future experimental directions. Evidence of glyoxal formation from ethylene ozonolysis might provide evidence of this underappreciated path in an important and long studied reaction system.


 Please wait while we load your content...
Please wait while we load your content...
