Electronic structure of triangular M3 (M = B, Al, Ga): nonclassical three-center two electron π bond and σ delocalization†
Abstract
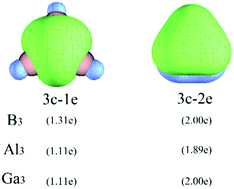
The small molecule clusters have received more and more attention due to their widespread applications in chemical insulators, explosives, semiconductors and the high energy density materials industry. The electron deficiency of group IIIA elements endows their clusters with interesting properties. In this work, the electronic structures of M3 (M = B, Al, Ga) have been investigated by means of a complete active space self-consistent field (CASSCF) method. The nature of the chemical bond has been analyzed using the quantum theory of atoms in molecules (QTAIM) and electron localization function (ELF) analyses. The following conclusions can be drawn: in M3 (M = B, Al, Ga) clusters, two π electrons are shared by three atoms forming a 3c–2e delocalization π bond. Going from B3 to Al3 to Ga3, more and more electrons move from the bond pair to the outside of the M atom, which leads to a gradual enhancement of the delocalization of σ electrons. Aromaticity and the adaptive natural density partitioning (AdNDP) analyses reveal the existence of the 3c–2e π bond and delocalization of σ electrons in the studied systems.


 Please wait while we load your content...
Please wait while we load your content...