PtN3-Embedded graphene as an efficient catalyst for electrochemical reduction of nitrobenzene to aniline: a theoretical study†
Abstract
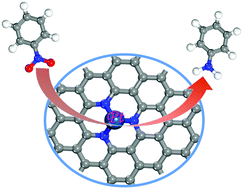
The electrochemical reduction of nitrobenzene (NBER) holds great promise for not only removing toxic pollutants, but also producing valuable aniline, in which the development of catalysts with high-efficiency still remains a huge challenge. In this work, by means of density functional theory (DFT) computations, we proposed several single transition metal (TM) atoms embedded into the single vacancy of graphene with nitrogen-doping (TMN3/G, TM = Ni, Cu, Pd, and Pt) as the catalysts for NBER. Our results revealed that, among these candidates, PtN3/G is the most active catalyst for the NBER due to its smallest limiting potential (−0.21 V), in which the hydrogenation of Ph-NO2* to Ph-NOOH* is identified as the potential-determining step. Compared with other catalysts, the strongest binding strength of Ph-NOOH* with PtN3/G is responsible for its superior catalytic activity towards NBER, which can be deeply understood on the basis of the corresponding electronic structure analysis. Thus, PtN3/G is a quite promising single-atom-catalyst with high efficiency for nitrobenzene reduction, which provides a rational paradigm for converting harmful nitrobenzene to valuable aniline under ambient conditions.


 Please wait while we load your content...
Please wait while we load your content...