Chlorination and tautomerism: a computational and UPS/XPS study of 2-hydroxypyridine ⇌ 2-pyridone equilibrium†
Abstract
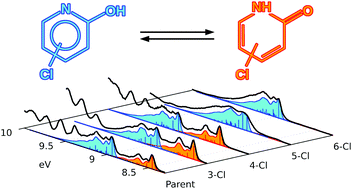
The prototropic tautomeric equilibrium in 2-hydroxypyridine serves as a prototype model for the study of nucleobases' behaviour. The position of such an equilibrium in parent and chlorine monosubstituted 2-hydroxypyridine compounds in the gas phase was determined using synchrotron based techniques. The lactim tautomer is dominant for the 5- and 6-substituted compounds, whereas the parent, 3- and 4-substituted isomers have comparable populations for both tautomers. Information was obtained by measuring valence band and core level photoemission spectra at the chlorine L-edge and carbon, nitrogen, and oxygen K-edges. The effect of chlorine on the core ionization potentials of the atoms in the heterocycle was evaluated and reasonable agreement with a simple model was obtained. Basic considerations of resonance structures correctly predicts the tautomeric equilibrium for the 5- and 6-substituted compounds. The vibrationally resolved structure of the low energy portion of the valence band photoionization spectra is assigned based on quantum-chemical calculations of the neutral and charged species followed by simulation of the vibronic structure. It is shown that the first ionization occurs from a π orbital of similar shape for both tautomers. In addition, the highly distinctive vibronic structure observed just above the first ionization of the lactim, for three of the five species investigated, is assigned to the second ionization of the lactam.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...