A DFT study for CO2 hydrogenation on W(111) and Ni-doped W(111) surfaces†
Abstract
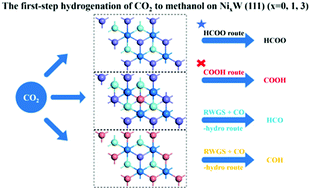
The first-step hydrogenation of CO2 to methanol via a HCOO route, COOH route, and RWGS + CO-hydro route on NixW(111) (x = 0, 1, 3) has been studied using density functional theory (DFT) calculations. CO2 and H could be chemically adsorbed on Ni-doped W(111) surfaces with relatively high adsorption energy, due to the synergistic effect of W that helps anchoring CO2 and Ni that facilitates the adsorption of H. The HCOO route is the main path for the first-step hydrogenation of CO2 with lower barriers on all three surfaces. Besides, competition between the HCOO route and RWGS + CO-hydro route could be enhanced with the increase in doped Ni on the W(111) surface. Furthermore, the first-step hydrogenation of CO2 hardly undergoes the COOH pathway because of the higher barriers, although the doping of Ni has slightly reduced the barrier of COOH formation. Our calculated results indicate that the W(111) and Ni-doped W(111) surface are potential candidate surfaces for CO2 hydrogenation to methanol, and Ni doping could influence the selectivity of reduction pathways.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...