Oxygen adsorption properties of small cobalt oxide clusters: application feasibility as oxygen gas sensors
Abstract
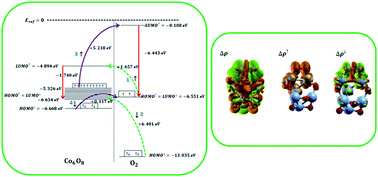
In this paper, a theoretical mechanism for oxygen adsorption on small-size cobalt oxide clusters is investigated. For this purpose, we employed dispersion-corrected density functional theory (DFT-D2). In this scheme, van der Waals interactions and the spin polarization mode are activated. Our calculations show the most stable oxygen adsorption configurations on the small-size thermodynamically stable cobalt oxide clusters, which are considered as (CoO)n (n = 2, 3, 4) and (Co3O4)n (n = 1, 2). The equilibrium geometries, adsorption energies, and electronic structures in terms of ionization potential, electron affinity, energy gap, spatial distribution of orbitals, partial density of states of the oxygen molecule, and charge transfer are calculated. Spin-distinct charge transfer is comprehensively studied employing schematic representations of energy levels along with the Lowdin charge analysis and visualization of charge density redistribution near the adsorption sites. Studies indicate that charge is totally transferred from cobalt oxide clusters to oxygen, which consists of spin-up charge transfer from oxygen to the clusters and spin-down charge transfer from the clusters to oxygen. It was seen that upon oxygen adsorption, the energy gap of the clusters increases and therefore conductivity decreases. Also, oxygen chemically adsorbs on the cobalt oxide clusters in an exothermic process. Therefore, oxygen molecules could be detected by pristine cobalt oxide clusters via conductometric and thermoelectric type sensors.


 Please wait while we load your content...
Please wait while we load your content...