Hydrogen adsorption on In2O3(111) and In2O3(110)†
Abstract
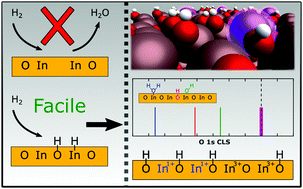
In2O3-Based catalysts have been measured to have a high activity for CO2 hydrogenation to H3COH. Here, we use density functional theory calculations with and without Hubbard-U corrections in combination with ab initio thermodynamics to investigate the dissociative adsorption of H2 over In2O3(111) and In2O3(110). H2 is found to dissociate heterolytically with a moderate barrier on both facets. Diffusion of hydrogen leads to the preferred homolytic adsorption configuration. Vacancy formation by water formation is thermodynamically preferred at high hydrogen coverages. Both surfaces are found to be hydroxylated at typical reaction conditions with the highest coverage predicted for In2O3(110). O 1s core level shifts are calculated for different coverages. The hydroxylated surfaces show two distinct shifts corresponding to different types of OH-groups. The presence of surface oxygen vacancies is not visible in the O 1s signatures. The results show that hydroxylation of the surfaces results in changes of the oxidation state of In-ions, which suggests that the redox properties on In2O3 are important for catalytic reduction of CO2 to added value chemicals.


 Please wait while we load your content...
Please wait while we load your content...