Defects and polaronic electron transport in Fe2WO6
Abstract
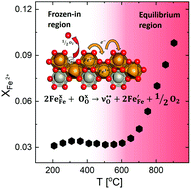
We report the synthesis of phase pure Fe2WO6 and its structural characterization by high quality synchrotron X-ray powder diffraction, followed by studies of electric and thermoelectric properties as a function of temperature (200–950 °C) and pO2 (1–10−3 bar). The results are shown to be in accordance with a defect chemical model comprising formation of oxygen vacancies and charge compensating electrons at high temperatures. The standard enthalpy and entropy of formation of an oxygen vacancy and two electrons in Fe2WO6 are found to be 113(5) kJ mol−1 and 41(5) J mol−1 K−1, respectively. Electrons residing as Fe2+ in the Fe3+ host structure act as charge carriers in a small polaron conducting manner. A freezing-in of oxygen vacancies below approximately 650 °C results in a region of constant charge carrier concentration, corresponding to an iron site fraction of XFe2+ ≅ 0.03. By decoupling of mobility from conductivity, we find a polaron hopping activation energy of 0.34(1) eV and a charge mobility pre-exponential u0 = 400(50) cm2 kV−1 s−1. We report thermal conductivity for the first time for Fe2WO6. The relatively high conductivity, large negative Seebeck coefficient and low thermal conductivity make Fe2WO6 an interesting candidate as an n-type thermoelectric in air, for which we report a maximum zT of 0.027 at 900 °C.

 Please wait while we load your content...
Please wait while we load your content...