An experimental and computational study on the material dispersion of 1-alkyl-3-methylimidazolium tetrafluoroborate ionic liquids†
Abstract
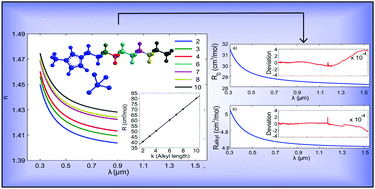
The material dispersion of the [Ckmim][BF4] (k = 2, 3, 4, 6, 7, 8, 10) family of ionic liquids is measured at several temperatures over a broad spectral range from 300 nm to 1550 nm. The experimental curves are fitted to a modified three-resonance Sellmeier model to understand the effects of temperature and alkyl chain length on the dispersion behaviour. From the parameters of the fitting, we analyze the influence that the different constituents of these ionic liquids have on the dispersion behaviour. In addition, a semi-empirical approach combining simulated electronic polarizabilities and experimental densities for predicting the material dispersion is successfully tested by using a direct comparison with the experimental results. The limitations of this method are analyzed in terms of the molecular structure of the ionic liquids. The results of this work aim to increase our knowledge about how the molecular structure of an ionic liquid influences its material dispersion. Understanding this influence is fundamental to producing ionic liquids with tailored optical properties.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...