Understanding ionic mesophase stabilization by hydration: a solid-state NMR study†
Abstract
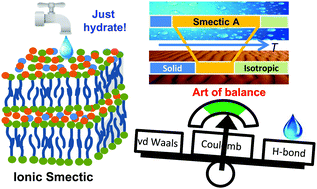
The correlation between the water contribution to hydrogen bonding within ionic sublayer, mesophase order parameter, and ion translational self-diffusion in the layered ionic liquid crystalline phase is investigated. Changes in hydrogen bonding, conformational and translational dynamics, and orientational order upon hydration were followed by solid-state NMR combined with density functional theory (DFT) analysis. We observed that the smectic mesophase of monohydrated imidazolium-based ionic liquids, which was stabilized in a wider temperature range compared to that of anhydrous materials, counterintuitively exhibited a lower orientational order of organic cations. Thus the role of anisotropic alignment of cations and contribution of dispersion forces in the mesophase stability decreased upon hydration. The local dynamics of cations is controlled by the alignment of the bulky methyl-imidazolium ring, experiencing strong electrostatic and H-bond interactions in the ionic sublayer. Anisotropy of translational diffusion increased in the hydrated samples, thus supporting the layer-stabilizing effect of water. The effect of decreasing molecular order is outweighed by the contribution of water hydrogen bonding to the overall interaction energy within the ionic sublayer.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...