Surface diffusion manifestation in electrodeposition of metal anodes†
Abstract
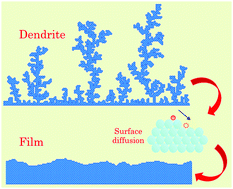
Metal anode-based battery systems have been deemed indispensable towards energy storage renaissance engendering extensive research into strategies countering dendritic growth of metal electrodeposition. Fundamentally, the morphological evolution of a material is uniquely characterized by the heights of its self-diffusion barrier across multiple pathways. Herein, based on a coarse-grained kinetic Monte Carlo method, we derive insights into the nucleation and growth of metallic electrodeposits in liquid electrolytes, governed by surface self-diffusion characteristics cognizant of the diverse diffusion routes including terrace, away from step and interlayer pathways. We deconvolve the roles played by each of these surface diffusion mechanisms in conjunction with the electrochemical reaction rate on the deposition morphology regime (film vs. mossy vs. fractal). We identify interlayer diffusion as the predominant morphology-determining mechanism; dendrite-free deposition even at moderate current rates constrains this diffusion barrier to an upper limit. Additionally, we highlight subtle features amidst the realm of the morphological growth assortment that connect to the cell's electrochemical performance. Finally, we delineate morphological features of Li, Na, Mg and Al based on their respective surface diffusion barriers and applied overpotentials, and provide a baseline for the interpretation of experimental observations. This fundamental study sheds light on the mesoscale underpinnings of morphological variances in mono-valent and multi-valent metal electrodeposition.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
