Reactivity and rotational spectra: the old concept of substitution effects†
Abstract
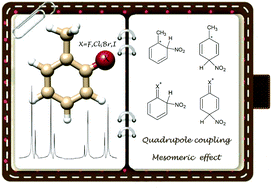
The internal rotation of methyl groups and nuclear quadrupole moments of the halogens Cl, Br, I in o-halotoluenes cause complex spectral fine and hyperfine structures in rotational spectra arising from angular momentum coupling. Building on the existing data regarding o-fluorotoluene and o-chlorotoluene, the investigations of o-bromotoluene and o-iodotoluene allow for a complete analysis of the homologous series of o-halogenated toluenes. The trend in the methyl barriers to internal rotation rising with the size of the halogen can be rationalised by repulsion effects as predicted by MP2 calculations. Furthermore, the analysis of the observed quadrupole coupling serves as a quantitative intra-molecular probe, e.g. for the explanation of the relative reaction yields in the nitration of halotoluenes, related to the different π-bond character of the C–X bond depending on the position of substitution.


 Please wait while we load your content...
Please wait while we load your content...