Performance of the asymptotic expansion method to derive equations of state for hard polyhedron fluids
Abstract
The asymptotic expansion method is used to derive analytical expressions for the equations of state of 14 hard polyhedron fluids such as cube, octahedron, rhombic dodecahedron, etc., by knowing the values of only the first eight virial coefficients. The results for the compressibility factor were compared with the most recent ones reported in the literature and obtained by computer simulations. Good results (averaged deviations below 1%) are found for the 8 fluids studied. On the other hand, the method seems to be inadequate, at least with the presently available values for the virial coefficients and compressibility factors, for 4 polyhedron fluids. Unfortunately, sometimes the method does not give low deviations at high densities or it gives excessively high values for the location of the pole. As an advantage, the value of the pole for the compressibility factor is always positive, which is not observed when other methods are used.
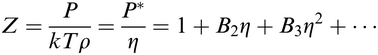


 Please wait while we load your content...
Please wait while we load your content...