Ru–polyoxometalate as a single-atom electrocatalyst for N2 reduction to NH3 with high selectivity at applied voltage: a perspective from DFT studies†
Abstract
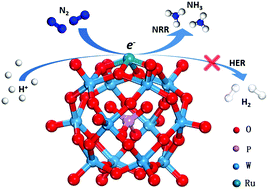
Many highly active electrocatalysts for the reduction of N2 to NH3 (NRR) have been synthesized but suffer from poor selectivity. One crucial reason is that the adsorption of hydrogen often dominates at the active centers at applied voltage, which leads to the competitive hydrogen evolution reaction. This work used density functional theory (DFT) calculations to develop a class of stable polyoxometalate-based electrocatalysts including phosphomolybdic-, phosphotungstic-, silicotungstic-, and silicomolybdic-acid supported Ru single atoms to efficiently catalyze the NRR process with an overpotential lower than 0.25 V. More importantly, phosphomolybdic- and phosphotungstic acid-supported Ru electrocatalysts can achieve high selectivity at applied voltage. This work offers useful insights into designing high-performance polyoxometalate-based electrocatalysts for the NRR.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...