A relationship between membrane permeation and partitioning of nitroaromatic explosives and their functional groups. A computational study†
Abstract
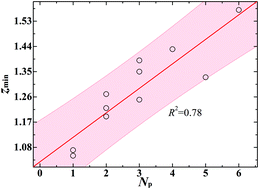
Nitroaromatic explosives, such as 2,4,6-trinitrotoluene, are representative aromatic compounds, which are generally highly toxic. For their toxic mechanisms, little is known about their interaction with cell membranes, although this is essential for their absorption, distribution, metabolism, excretion and toxicity profiling. Here, we investigated the membrane permeation and partitioning of 12 nitroaromatic explosives with typical functional groups (e.g., –NO2, –NH2, –OCH3 and –OH) by all-atom molecular dynamics simulations. Based on free-energy curves, we obtained three key parameters that describe the behavior of permeation and partitioning, namely liposome–water partition coefficient (KLW), permeability coefficient (P) and translocation time (τ). Functional groups contribute little to KLW, indicating that the membrane absorption of nitroaromatic explosives is primarily controlled by the hydrophobic effect of the benzene ring. P shows an obvious decline with increasing polar group number (Np), and therefore τ exhibits a continuous increase. In addition, the preferred location (zmin) of explosive molecules in membranes is closer to the head group of lipids when they have more polar groups. Further analysis shows that the hydrogen bond (H-bond) interaction of explosives with water and lipids plays a crucial role in the dependence of permeation and partitioning on polar groups. The molecules with larger Np can form more H-bonds with water in the aqueous phase, which limits their motion into the deeper hydrophobic region of membranes. Moreover, the desolvation/loss of H-bonds leave zmin controls the membrane permeation properties, which is also correlated with Np. The work reveals the physical essence of the relationship between the membrane permeation and partitioning of nitroaromatic explosives and their functional groups. These results may be also applicable to other (e.g., polycyclic) nitroaromatic compounds.


 Please wait while we load your content...
Please wait while we load your content...