Chemical potentials of electric double layers at metal–electrolyte interfaces: dependence on electrolyte concentration and electrode materials, and application to field-effect transistors†
Abstract
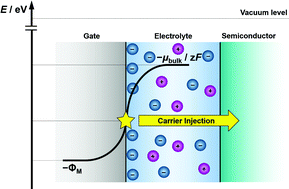
When a metal is soaked in an electrolyte solution, the metal and solution affect each other through the formation of electric double layers (EDLs) at their interfaces. The EDLs at metal–electrolyte interfaces can realize high-density charge-carrier injections and accumulations, and thus have recently attracted attention for their potential application to energy storage, and electronic and electrochemical devices. In such EDL-based devices, including field-effect transistors (FETs), the potential energy of surface electrons in the metal electrodes (EM) governs the transistor device performance. This is in clear contrast to redox-driven electrochemical devices such as dye-sensitized solar cells and electrochromic devices, whose performance is primarily governed by the potentials of the redox-active species. However, there has been no systematic research to bridge the distance between metal electrons and electrolyte ions. In the present study, we carefully examined the dependence of EM of ITO, Au and Pt electrodes on the concentration of the PEG solutions of LiCl and MgCl2, because it has been well established that the chemical potential of electrolyte solutions is dependent on the solution concentrations. Our results showed that, at the same electrolyte concentration, the values of EM increased in the order of ITO, Au and Pt; moreover, on the same electrode, EM showed linear decreases as a function of the logarithm of the electrolyte concentrations. To understand these behaviors, we developed a theoretical treatment of the EDLs based on the simple Gouy–Chapman model, and obtained the theoretical expressions of EM in terms of the concentration of electrolyte and the work function of the metal electrode (ΦM), which were found to successfully explain the dependences of EM on the electrolyte concentration and the electrode materials. We also examined the EDL-FETs of platinum phthalocyanine (PtPc), with various LiCl–PEG solutions of different concentrations as gate electrolytes. The threshold voltage eVT and EM exhibited a linear relation, which was well explained by the relation between EM and the valence band energy EVB of PtPc. The transfer characteristics at various gate voltage VG were found to be well normalized by a function of eVG + EM.


 Please wait while we load your content...
Please wait while we load your content...