Theoretical study of the reaction mechanism and kinetics of the phenyl + propargyl association†
Abstract
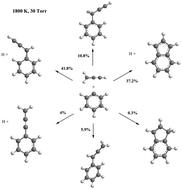
Potential energy surface for the phenyl + propargyl radical recombination reaction has been studied at the CCSD(T)-F12/cc-pVTZ-f12//B3LYP/6-311G** level of theory for the closed-shell singlet species and at the triplet–singlet gap CASPT2/cc-pVTZ-CCSD(T)-F12/cc-pVTZ-f12//CASSCF/cc-pVTZ level of theory for the diradical species. High-pressure limit rate constants for the barrierless channels were evaluated with variable reaction coordinate transition state theory (VRC-TST). Rice–Ramsperger–Kassel–Marcus Master Equation (RRKM-ME) calculations have been performed to assess temperature- and pressure-dependent phenomenological rate constants and product branching ratios. The entrance channels of the radical association reaction produce 3-phenyl-1-propyne and phenylallene which can further dissociate/isomerize into a variety of unimolecular and bimolecular products. Theoretical evidence is presented that, at combustion relevant conditions, the phenyl + propargyl recombination provides a feasible mechanism for the addition of a second five-member ring to the first six-member aromatic ring producing the prototype two-ring species indene and indenyl. Rate expressions for all important reaction channels in a broad range of temperatures and pressures have been generated for kinetic modeling.


 Please wait while we load your content...
Please wait while we load your content...
