Why does B2O3 suppress nepheline (NaAlSiO4) crystallization in sodium aluminosilicate glasses?†
Abstract
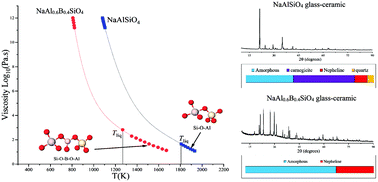
The uncontrolled growth of nepheline (NaAlSiO4) crystals during the manufacturing of sodium aluminosilicate glasses via the fusion draw or float techniques and during the vitrification of some of the sodium- and alumina-rich nuclear waste glasses is a well-known problem. The addition of B2O3 to suppress the crystallization in these glasses is well documented in the literature. Another advantage of B2O3 is that it lowers the viscosity of the glass melt and, if incorporated in its trigonal coordination state, will improve the intrinsic damage resistance of the final glass product. Hence, B2O3 has been an integral component of glass compositions for advanced industrial applications and for nuclear waste vitrification. However, one major disadvantage of adding B2O3 to alkali aluminosilicate based glasses is its adverse impact on their chemical durability due to the rapid hydrolysis of B[3,4]–O–B[3,4] bonds in comparison to (Si, Al)–O–(Si, Al) bonds. Therefore, designing a boron-containing alkali aluminosilicate based functional glass with minimal tendency towards crystallization and high chemical durability requires an in-depth fundamental understanding of the mechanism through which B2O3 tends to suppress crystallization in these glasses. There is no current consensus on the fundamental mechanism through which B2O3 tends to suppress nepheline crystallization in these glasses. Based on the mechanisms described and the questions raised in the preceding literature, the present study focuses on addressing the ongoing debate through a detailed structural and thermo-kinetic investigation of glasses designed in the Na2O–Al2O3–B2O3–SiO2 based quaternary system over a broad composition space. Using a combination of Raman and (1D and 2D) nuclear magnetic resonance spectroscopies along with equilibrium and non-equilibrium viscosity, and liquidus temperature measurements, it has been shown that the substitution of Si–O–Al by Si–O–B linkages in the glass structure results in a significant increase in the glass forming ability as well as an increase in the liquidus viscosity (slower diffusivity), thereby suppressing the nepheline crystallization.


 Please wait while we load your content...
Please wait while we load your content...
