Precursor chemistry of h-BN: adsorption, desorption, and decomposition of borazine on Pt(110)†
Abstract
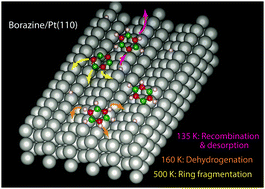
Adsorption, desorption and fragmentation of borazine on Pt(110) are studied by temperature-programmed desorption, ultraviolet photoemission spectroscopy, workfunction measurements and density functional theory. Borazine adsorbs in part dissociatively, forming an upright (B3N3H5˙)ads adsorption complex. Radicals with a N–Pt bond are weakly bound and desorb recombinatively following second-order kinetics. Radicals with a B–Pt bond are similar in binding strength to the molecularly adsorbed species, which binds through dispersive forces to the (111) facets of the (1 × 2) reconstructed Pt(110). Both do not desorb but are dehydrogenated beyond T = 150 K. As T approaches 600 K the B–N ring progressively breaks down into its atomic constituents. The borazine ice multilayer is capable of trapping significant amounts of hydrogen. Previous studies of borazine adsorption on other transition metal surfaces yield a very similar pattern. Reported multiple molecular desorption peaks are artefacts. Implications for the nucleation and growth of h-BN monolayers at high temperatures are discussed.


 Please wait while we load your content...
Please wait while we load your content...