Hydration structure and water exchange kinetics at xenotime–water interfaces: implications for rare earth minerals separation†‡
Abstract
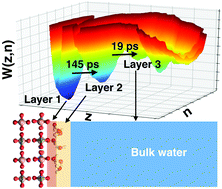
Hydration of surface ions gives rise to structural heterogeneity and variable exchange kinetics of water at complex mineral–water interfaces. Here, we employ ab initio molecular dynamics (AIMD) simulations and water adsorption calorimetry to examine the aqueous interfaces of xenotime, a phosphate mineral that contains predominantly Y3+ and heavy rare earth elements. Consistent with natural crystal morphology, xenotime is predicted to have a tetragonal prismatic shape, dominated by the {100} surface. Hydration of this surface induces multilayer interfacial water structures with distinct OH orientations, which agrees with recent crystal truncation rod measurements. The exchange kinetics between two adjacent water layers exhibits a wide range of underlying timescales (5–180 picoseconds), dictated by ion–water electrostatics. Adsorption of a bidentate hydroxamate ligand reveals that {100} xenotime surface can only accommodate monodentate coordination with water exchange kinetics strongly depending on specific ligand orientation, prompting us to reconsider traditional strategies for selective separation of rare-earth minerals.


 Please wait while we load your content...
Please wait while we load your content...
