A long lasting sunscreen controversy of 4-aminobenzoic acid and 4-dimethylaminobenzaldehyde derivatives resolved by ultrafast spectroscopy combined with density functional theoretical study†
Abstract
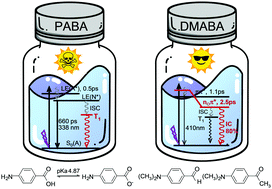
4-Aminobenzoic acid (PABA) is one of the earliest patented and most commonly used sunscreen components. There is however a long-lasting controversy on its photo-protective efficacy owing to the lack of information on its protolytic equilibrium and photo-dynamics after absorption of ultraviolet radiation in physiologically relevant aqueous solution. The excitation dynamics in water also remains largely unknown for analogs of PABA such as 4-dimethylaminoacetophenone (DMAAP) and 4-dimethylaminobenzaldehyde (DMABA) which are recognized as prototypes for photo-induced twisted intramolecular charge transfer (TICT). Herein we report a combined application of femtosecond broadband time-resolved fluorescence and transient absorption coupled with density functional theoretical study for PABA, DMAAP, and DMABA under several solvent conditions with representative properties in terms of the pH, polarity and hydrogen bonding capacity. The results we gained demonstrate that, in a neutral aqueous solution, PABA taking the deprotonated anion form in the ground state undergoes rapid protonation after excitation, producing excited state species in the neutral form that may shift effectively by intersystem crossing (ISC) to the long-lasting triplet state capable of damaging nucleic acids. This provides evidence at the molecular level for the detrimental effect of PABA if used as a sunscreen ingredient. In contrast, our investigation on DMAAP and DMABA unveils an unusual solvent controlled deactivation dynamics rendered by the participation of the carbonyl oxygen associated nOπ* state featuring energy and structure strongly responsive to solvent properties. In particular, these molecules in water exhibit solute–solvent hydrogen bonding at the sites of the carbonyl oxygen and the amino nitrogen which is, respectively, weakened and strengthened after the excitation, leading to state reversal and formation of a nOπ* state with a peculiar non-planar structure. This quenches strongly the excitation, eliminates the TICT, suppresses the ISC and opens up the otherwise inaccessible internal conversion (IC) to account for ∼80% of the entire deactivation. The IC, observed to proceed at a rate of ∼2.5 ps, allows the effective recovery of the ground state, providing substantial protection against ultraviolet irradiation. Moreover, the revelation of highly solvent sensitive fluorescence emission from DMABA and DMAAP implies the potential application of these molecules as the functional element in the design of sensory materials for probing the polarity and hydrogen bonding character of the surrounding environment.


 Please wait while we load your content...
Please wait while we load your content...