Growth and auto-oxidation of Pd on single-layer AgOx/Ag(111)†
Abstract
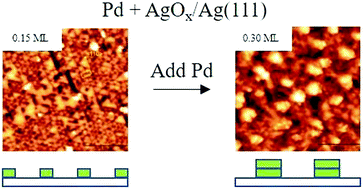
We investigated the growth and auto-oxidation of Pd deposited onto a AgOx single-layer on Ag(111) using scanning tunneling microscopy (STM) and X-ray photoelectron spectroscopy (XPS). Palladium initially grows as well-dispersed, single-layer clusters that adopt the same triangular shape and orientation of Agn units in the underlying AgOx layer. Bi-layer clusters preferentially form upon increasing the Pd coverage to ∼0.30 ML (monolayer) and continue to develop until aggregating and forming a nearly conformal Pd bi-layer at a coverage near 2 ML. Analysis of the STM images provides quantitative evidence of a transition from single to bi-layer Pd growth on the AgOx layer, and a continuation of bi-layer growth with increasing Pd coverage from ∼0.3 to 2 ML. XPS further demonstrates that the AgOx layer efficiently transfers oxygen to Pd at 300 K, and that the fraction of Pd that oxidizes is approximately equal to the local oxygen coverage in the AgOx layer for Pd coverages up to at least ∼0.7 ML. Our results show that oxygen in the initial AgOx layer mediates the growth and structural properties of Pd on the AgOx/Ag(111) surface, enabling the preparation of model PdAg surfaces with uniformly distributed single or bi-layer Pd clusters. Facile auto-oxidation of Pd by AgOx further suggests that oxygen transfer from Ag to Pd could play a role in promoting oxidation chemistry of adsorbed molecules on PdAg surfaces.

 Please wait while we load your content...
Please wait while we load your content...
