Absorption spectra of benzoic acid in water at different pH and in the presence of salts: insights from the integration of experimental data and theoretical cluster models†
Abstract
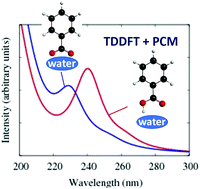
The absorption spectra of molecular organic chromophores in aqueous media are of considerable importance in environmental chemistry. In this work, the UV-vis spectra of benzoic acid (BA), the simplest aromatic carboxylic acid, in aqueous solutions at varying pH and in the presence of salts are measured experimentally. The solutions of different pH provide insights into the contributions from both the non-dissociated acid molecule and the deprotonated anionic species. The microscopic interpretation of these spectra is then provided by quantum chemical calculations for small cluster models of benzoic species (benzoic acid and benzoate anion) with water molecules. Calculations of the UV-vis absorbance spectra are then carried out for different clusters such as C6H5COOH·(H2O)n and C6H5COO−·(H2O)n, where n = 0–8. The following main conclusions from these calculations and the comparison to experimental results can be made: (i) the small water cluster yields good quantitative agreement with observed solution experiments; (ii) the main peak position is found to be very similar at different levels of theory and is in excellent agreement with the experimental value, however, a weaker feature about 1 eV to lower energy (red shift) of the main peak is correctly reproduced only by using high level of theory, such as Algebraic Diagrammatic Construction (ADC); (iii) dissociation of the BA into ions is found to occur with a minimum of water molecules of n = 8; (iv) the deprotonation of BA has an influence on the computed spectrum and the energetics of the lowest energy electronic transitions; (v) the effect of the water on the spectra is much larger for the deprotonated species than for the non-dissociated acid. It was found that to reproduce experimental spectrum at pH 8.0, additional continuum representation for the extended solvent environment must be included in combination with explicit solvent molecules (n ≥ 3); (vi) salts (NaCl and CaCl2) have minimal effect on the absorption spectrum and; (vii) experimental results showed that B-band of neutral BA is not sensitive to the solvent effects whereas the effect of the water on the C-band is significant. The water effects blue-shift this band up to ∼0.2 eV. Overall, the results demonstrate the ability to further our understanding of the microscopic interpretation of the electronic structure and absorption spectra of BA in aqueous media through calculations restricted to small cluster models.


 Please wait while we load your content...
Please wait while we load your content...
