Selective formation of molecular junctions with high and low conductance states by tuning the velocity of electrode displacement†
Abstract
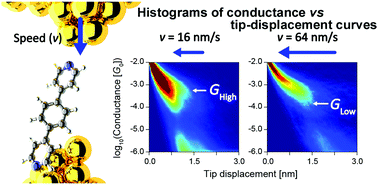
A single-molecule junction of 1,4-di(4-pyridyl)benzene (DPB) was prepared in a nano-gap between two Au electrodes using the scanning tunnelling microscopy-based break junction method (STM-BJ). Electric conductance and current versus bias voltage (I−V) measurements during the pulling and pushing processes of DPB single-molecule junctions revealed that high (H) and low (L) conductance states formed in both the pulling and pushing processes. Analysis of the I−V curves based on a single-level model indicated that the difference in conductivity of the H and L states mainly arises from high and low metal–molecule electric coupling in the junction. We demonstrated the controllable formation of H and L conductance states by simply tuning the velocity of electrode displacement in the pushing process. In the pulling process, both H and L states formed regardless of the velocity (v) of electrode displacement, while in the pushing process, H and L states could be selectively fabricated by using low (v = 16 nm s−1) and high (v = 64 nm s−1) velocities of displacement, respectively. This study provides a simple approach to selectively fabricate high and low conductance states by fine tuning of the electrode displacement.


 Please wait while we load your content...
Please wait while we load your content...
