Hydrogen tunnelling in the rearrangements of carbenes: the role of dynamical calculations†
Abstract
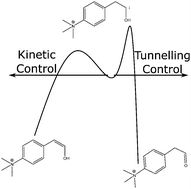
Tunnelling controlled chemical reactions are those which preferably proceed through pathways with high but narrow potential energy barriers, via quantum tunnelling, resulting in a product that would be disfavoured classically. These reactions are very sensitive to barrier width, height and temperature and so dynamical theoretical methods are required to describe these processes. Recent experimental work on charge-tagged phenyl pyruvic acid derivatives has found, in contrast to similar systems, no evidence of tunnelling control. Using semiclassical transition state theory, we rationalise these results and find tunnelling is significant in this system.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...