Hydrogen abstraction in astrochemistry: formation of ˙CH2CONH2 in the reaction of H atom with acetamide (CH3CONH2) and photolysis of ˙CH2CONH2 to form ketene (CH2CO) in solid para-hydrogen†
Abstract
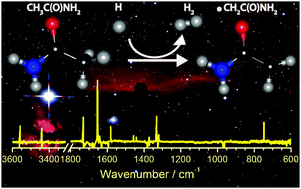
Acetamide (CH3CONH2) is the largest molecule containing an amide bond that has been detected in an interstellar medium; it is considered to be a precursor for complex organic molecules (COM). We utilized the advantages of a para-hydrogen (p-H2) quantum-solid matrix host to perform efficient reactions of hydrogen atoms with CH3CONH2. The H-abstraction reaction from the methyl group of CH3CONH2 to produce the 2-amino-2-oxoethyl radical, ˙CH2CONH2, was observed as the sole reaction channel in solid p-H2 at 3.3 K, consistent with theoretical predictions that this reaction has the smallest barrier among all possible channels. Our results show that the amide bond of acetamide is unaffected by hydrogen exposure, but the hydrogen abstraction activates this molecule to react with other species on its methyl site to extend its size or to include other functional groups as a first step to form COM under prebiotic or abiotic conditions. This previously neglected path should be considered in the astrochemical modeling. The photolysis of ˙CH2CONH2 at wavelengths 380–450 nm produces ketene; this step might provide a plausible mechanism to explain the anti-correlated abundance of ketene and acetamide in some astronomical observations.

 Please wait while we load your content...
Please wait while we load your content...