Is basicity the sole criterion for attaining high carbon dioxide capture in deep-eutectic solvents?†
Abstract
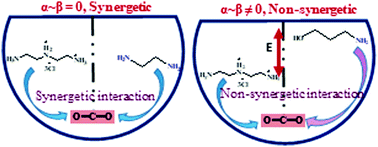
A critical analysis of the role of Hammett basicity (H−) and aqueous basicity (pKa) in CO2 uptake in deep-eutectic solvents (DESs) suggests that neither H− nor pKa correlates with the CO2 w/w% capacity in the studied DESs. Instead, strong “synergistic interactions” between donor and acceptor moieties satisfactorily relate to the w/w% of CO2 in DESs.


 Please wait while we load your content...
Please wait while we load your content...