Correlation between mobility and the hydrogen bonding network of water at an electrified-graphite electrode using molecular dynamics simulation†
Abstract
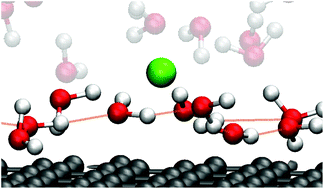
Focusing on the electric double layer formed at aqueous solution/graphite electrode interfaces, we investigated the relationship between the mobility of interfacial water and its hydrogen bonding networks by using molecular dynamics simulations. We focused on the mobility of the first hydration layer constructed nearest to the electrode. The mobility was determined by calculating the diffusion coefficient which showed an opposite trend to that of the applied potential polarity. The mobility decreased upon positive potentials while showing an increase upon negative potentials, which is rationalized by the strength of the interfacial hydrogen bonding networks.


 Please wait while we load your content...
Please wait while we load your content...