New aspects of C2 selectivity in electrochemical CO2 reduction over oxide-derived copper†
Abstract
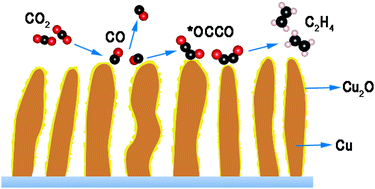
The electrocatalytic CO2 reduction to yield C2 products is of particular interest in solar-to-fuel conversion schemes. The nanocrystalline oxide derived copper (ODCu) electrodes are specifically attractive due to their high faradaic efficiency towards C2 hydrocarbons like ethylene, ethane, acetate and ethanol. However, the mechanistic understanding of this special selectivity is still an impediment. In this work, ODCu is obtained from Cu2O nanowires and employed for electrocatalytic CO2 reduction, during which ethylene is found to be the major product with a faradaic efficiency of 65% at −0.8 V (vs. RHE). By in situ photoresponse measurement, combined with the ex situ structure and composition analysis, Cu2O is demonstrated to be persistent on the surface of ODCu throughout the CO2 reduction reaction (CO2RR) even at high applied bias (−1.0 V vs. RHE), while Cu2O is not present on the bulk Cu foil. Density functional theory calculations are employed to further investigate the correlation between the surface Cu2O on ODCu and its C2 selectivity performance, which is attributed to the orbital interactions between the persistent oxide and CO2 reduction intermediates. It should be noted that uncovering the active sites is the initial step to understand the surface reaction chemistry in CO2RR; here, we propose that the presence of Cu2O is the key for C2 selectivity during CO2RR in the ODCu system, which may facilitate the development of highly efficient catalysts.


 Please wait while we load your content...
Please wait while we load your content...