Deeply coloured and highly fluorescent dipolar merocyanines based on tricyanofuran†
Abstract
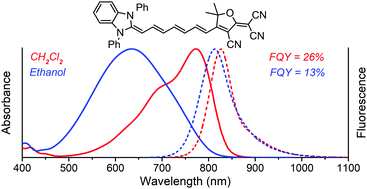
Three vinylogous series of merocyanines with the tricyanofuran (TCF) acceptor and different electron-donor groups have been synthesized, and their absorption and fluorescence spectra have been studied in different polarity solvents to reveal the polyene–polymethine transitions of their electronic structures. The TCF dyes with the indole or benzimidazole donor groups have relatively bright fluorescence in the red and near-IR spectral ranges. From the obtained data, the electron-acceptor ability of the TCF group has been estimated to be close to that of the thiobarbituric residue, while the TCF group is less prone to formation of solute–solvent H-bonds in protic media. From the DFT and TD-DFT calculations of the TCF-based dyes, performed using B3LYP, CAM-B3LYP, and M06-2X hybrid functionals, it has been revealed that their two major conformers have very close spectral-fluorescence properties, which explains why they are undistinguishable in the steady-state electronic spectra. However, the calculated polarizabilities and the first hyperpolarizabilities are more dependent on the molecular geometry, with both parameters being greater for the transoid conformer.
- This article is part of the themed collection: 2020 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...